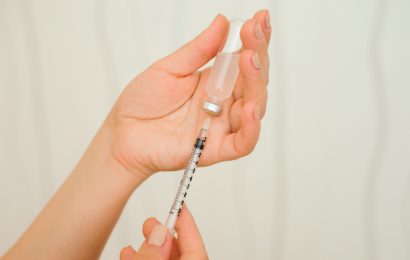
On September 8, pharmaceutical manufacturer Novo Nordisk issued a voluntary nationwide recall of six batches of its GlucaGen HypoKit due to two customer reports in Europe of detached needles on the syringe containing Sterile Water for Injection (SWFI). The GlucaGen HypoKit delivers glucagon, a hormone that works against the action of insulin and that is used to reverse severe hypoglycemia (low blood sugar).
The recalled batches were distributed starting February 15, 2016 and are as follows:
• Batch: FS6X270, Expiry: 09/30/2017
• Batch: FS6X296, Expiry: 09/30/2017
• Batch: FS6X538, Expiry: 09/30/2017
• Batch: FS6X597, Expiry: 09/30/2017
• Batch: FS6X797, Expiry: 09/30/2017
• Batch: FS6X875, Expiry: 09/30/2017
The batch number can be found on the upper right-hand side of the GlucaGen HypoKit box.
A company investigation suggested that 0.006% of the needles could potentially detach from the syringe in devices distributed since February 15, 2016, translating to approximately 4 of the 71,215 kits being recalled.
Customers who have a product from the recalled batches should call Novo Nordisk, toll free, at (888) 840-1137 from Monday to Friday between 8:30 AM and 6:00 PM Eastern Time to find out how to return the kit. The company will provide reimbursement for out-of-pocket costs with proof of purchase. Those who received a GlucaGen HypoKit through the Novo Nordisk Patient Assistance Program will receive a replacement.
Novo Nordisk is notifying all distributors by letter and phone and is arranging for the return of all the recalled kits.
Any adverse reactions or problems relating to the use of this product should be reported to MedWatch, the FDA’s safety and adverse event reporting program, by telephone at (800) FDA-1088 (332-1088), by fax at (800) FDA-1078 (332-1078), on this webpage, with the postage-paid FDA form 3500 (available here), or by mail to MedWatch, 5600 Fishers Lane, Rockville, Maryland 20852-9787.
If your GlucaGen HypoKit is not from the batches listed above, it is not subject to the recall and may be used as prescribed.
For more information, see the recall notification on the FDA’s website.
If you have Type 1 diabetes who loves the outdoors, you may be interested in the upcoming SoCal Slipstream Weekend. Bookmark DiabetesSelfManagement.com and tune in tomorrow to learn more.